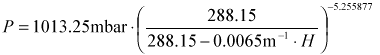Atmospheric noble gases 1
He, Ne, Ar, Kr, Xe, and Rn are called noble gases (or inerrt
gases) because of their inactivity in chemical reactions. A nice
summary of their discovery and history can be found here.
Abundance
Most aqueous systems are dominated by atmospheric noble gases:
- atmospheric abundance of noble gases (Tab)
- atmospheric isotope ratios (Tab)
noble gases concentrations in water are a function of temperature and
salinity
- they are often expressed as Bunsen coefficient (ccSTP of gas
dissolved
per cc of water at 1 atmosphere of pressure of the gas at a given
temperature) (Fig)
Early studies of atmospheric noble gases in isotope hydrology
- Oana (1957) studied the noble gas concentrations in rainwater,
groundwater,
and surface water and found that rainwater showed a seasonal variation,
while groundwater did not (Fig)
- Mazor (1972) suggested that paleoclimate information may be
contained
in
aquifers
- the first paleotemperature record was obtained by Andrews and Lee
from
an aquifer in the UK (Fig)
- they found a difference between the noble gas temperature of
Holocene
and
Glacial groundwater samples of about 6oC and that there
apparently
was no recharge during the last glacial maximum
- Heaton et al. (1981) measured N2 and Ar on surface
waters
and
groundwaters and discovered 'excess air' (fig)
Processes in the recharge area or in surface water bodies
Solubility equilibrium
- the initial condition for the noble gases is set at the water
table (fig):
C = b (T,S) * P/Po
- b is the Bunsen coefficient as a
function of
temperature T and salinity S, P is the partial pressure of the noble
gas
at the water table, Po = 1013.25 mbar
- noble gas concentrations as a function of T (absolute
(Fig),
relative (Fig) and salinity
(Fig))
- equations describing the concentrations of noble gases dissolved
in
water (in ccSTP/g) as a function of temperature often
have the form (example for Ar, atmospheric pressure, 100% relative
humidity):
- CAr = 0.001 *
EXP(-178.1725+251.8139*100/(273.15+T)+145.2337*
LN((273.15+T)/100)-22.2046*(273.15+T)/100)
- for equilibration at a different altitude, the concentrations
need to be corrected by the equivalent drop in pressure.
- for US Standard atmosphere:
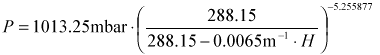
- CAr'(h) = CAr(0)*(P-PH2O)/(Po-PH2O)
- biological activity
- partial pressures of O2 and CO2 can
change
but often do so in a complementary way
- gravitational separation
- heavier isotopes enriched at bottom of a column
- thermal diffusion (due to temperature gradient)
- water vapor flux fractionation (gradient in relative humidity
near the
surface) , Severinghaus et al., 1995)
<>the last three effects working together (fig, Severinghaus et
al., 1995)
Excess air formation
- Xe vs Ne for two aquifers show fractionated and unfractionated
excess
aire
relative to air (fig)
- processes near the water table (fig)
- dissolution of additional gas due to partial or total
dissolution of
bubbles
- possibility for secondary gas exchange
- solubility controlled (fig)
or
diffusion controlled (fig)
secondary
equilibration
Resources
- Ozima, M and F. A. A. Podosek (2002) Noble gas geochemistry,
Cambridge University Press
- Aeschbach-Hertig, W., Peeters, F., Beyerle, U., and Kipfer, R.
(1999). Interpretation of dissolved atmospheric noble gases in natural
waters. Water Resources Research 35, 2779-2792.
- Stute, M., and Schlosser, P. (2000). Atmospheric noble gases. In
"Environmental tracers in subsurface hydrology" (P. G. Cook and A. L.
Herczeg, eds.), pp. 349-377. Kluwer, Boston.